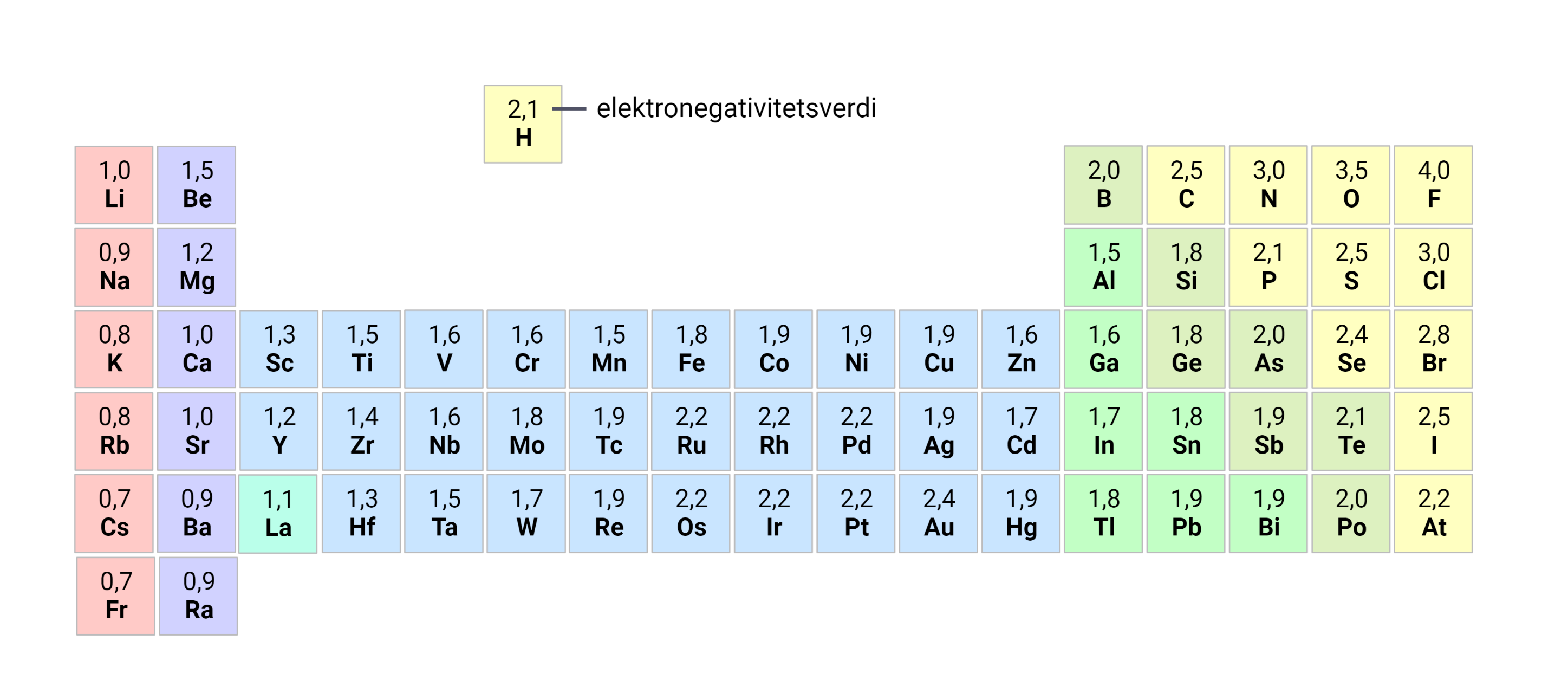
Breaking Down the Electronegativity Trend: A Game-Changer in Science – Electronegativity, a fundamental idea in chemistry, serves as a cornerstone in know-how chemical bonding and reactivity. this newsletter goals to delve into the intricacies of the electronegativity trend, exploring its significance, variations, and actual-world applications.

Introduction to Electronegativity
Electronegativity refers to the ability of an atom to draw electrons in the direction of itself in a chemical bond. It plays a pivotal role in figuring out the nature of bonds fashioned among atoms, thereby influencing the properties and behavior of molecules.
Understanding the Electronegativity Trend
The electronegativity of elements well-knownshows a predictable fashion across the periodic table. typically, electronegativity will increase from left to right and decreases from pinnacle to bottom inside a duration or group, respectively.
Factors Affecting Electronegativity
Several elements have an impact on the electronegativity of an atom, including its atomic length, nuclear rate, and electron protecting. large atoms with more defensive experience weaker nuclear enchantment, resulting in decrease electronegativity.
Importance in Chemical Bonding
Electronegativity dictates the form of chemical bonds shaped between atoms. In ionic bonds, electrons are transferred from one atom to every other because of a enormous distinction in electronegativity. Conversely, covalent bonds get up from the sharing of electrons among atoms with comparable electronegativities.
Electronegativity’s Role in Predicting Bond Polarity
The disparity in electronegativity among atoms in a bond determines its polarity. Polar bonds arise whilst there’s an uneven distribution of electrons, leading to partial costs on the atoms concerned. Nonpolar bonds, however, exhibit same sharing of electrons and no internet price.
Real-world Applications of Electronegativity
The concept of electronegativity unearths programs in numerous fields, inclusive of biology and industry. In biological structures, electronegativity influences the structure and function of molecules along with proteins and DNA. furthermore, industries make use of electronegativity standards in procedures which includes electroplating and corrosion prevention.
Historical Perspective on Electronegativity
The perception of electronegativity has evolved over time, with contributions from outstanding scientists together with Linus Pauling and Robert Mulliken. Their efforts laid the inspiration for modern-day understandings of chemical bonding and periodic trends.
Modern Methods of Determining Electronegativity
numerous scales exist for quantifying electronegativity, together with the Pauling scale and the Mulliken scale. those strategies depend on empirical records and theoretical fashions to assign numerical values to factors.
Exceptions to the Electronegativity Trend
Even as the electronegativity fashion commonly holds actual, there are exceptions and anomalies in the periodic table. Transition metals, as an instance, might also showcase variable electronegativities due to complicated electronic configurations.
Electronegativity and Reactivity
Electronegativity impacts the reactivity of elements and compounds. exceptionally electronegative factors generally tend to form stronger bonds and show off extra chemical interest, impacting their behavior in various reactions.
Impact on Physical Properties
The electronegativity of atoms impacts their physical residences, which include melting and boiling factors. elements with higher electronegativities typically have more potent intermolecular forces, leading to better melting and boiling points.
Electronegativity and Molecular Geometry
In molecular compounds, electronegativity influences the spatial association of atoms, main to awesome molecular shapes. Molecules with polar bonds regularly showcase asymmetrical geometries, affecting their properties and interactions.
Challenges and Controversies in Electronegativity
Regardless of its extensive use, electronegativity stays a subject of debate and scrutiny inside the scientific community. Critics point to obstacles in existing scales and the want for extra particular techniques of dimension.
Future Directions in Electronegativity Research
Advancements in computational chemistry and experimental techniques retain to beautify our understanding of electronegativity. destiny research goals to refine existing fashions and discover new applications in fields including materials technology and catalysis.
Conclusion
The electronegativity fashion serves as a fundamental precept in chemistry, offering insights into the behavior of atoms and molecules. by means of elucidating the factors that have an effect on electronegativity and its implications in diverse contexts, scientists can similarly extend our know-how of chemical bonding and reactivity.
Unique FAQs
- How does electronegativity fluctuate from electron affinity?
- Electronegativity measures an atom’s capacity to draw electrons in a chemical bond, even as electron affinity refers to the strength launched when an atom gains an electron.
- Are there any factors with 0 electronegativity?
- No, all factors exhibit some diploma of electronegativity, even though it could be noticeably low for noble gases.
- Can electronegativity values be terrible?
- Electronegativity values are generally positive, but they can be bad for positive theoretical calculations or individual chemical species.
- What function does electronegativity play in determining solubility?
- Electronegativity impacts the polarity of molecules, which, in turn, affects their solubility in polar or nonpolar solvents.
- How do isotopes of the identical element fluctuate in electronegativity?
- Isotopes of the same detail have identical chemical houses, inclusive of electronegativity, as they possess the equal number of protons and electrons.